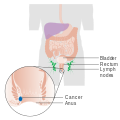
Anal cancer
| Anal cancer | |
|---|---|
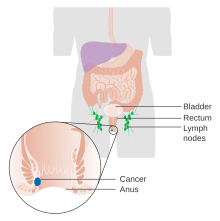 | |
| Diagram showing stage 1 anal cancer | |
| Specialty | Oncology |
| Symptoms | Anal bleeding or lump[1] |
| Usual onset | Age over 45 years[2] |
| Types | Squamous cell carcinoma, adenocarcinoma, small cell carcinoma, melanoma[3] |
| Risk factors | Human papillomavirus (HPV), HIV/AIDS, receptive anal sex, smoking, many sexual partners[1][4] |
| Diagnostic method | Physical examination, tissue biopsy[1] |
| Differential diagnosis | Anal warts, hemorrhoids, anal fissure[5] |
| Prevention | HPV vaccination, avoiding risk factors[6] |
| Treatment | Radiation therapy, chemotherapy, surgery[1] |
| Prognosis | Five year survival ~68% (US 2015)[2] |
| Frequency | 8,300 (US 2019)[2] |
| Deaths | 1,280 (US 2019)[2] |
Anal cancer is a cancer which arises from the anus, the distal opening of the gastrointestinal tract.[1] Symptoms may include bleeding from the anus or a lump near the anus.[1] Other symptoms may include pain, itchiness, or discharge from the anus.[1] A change in bowel movements may also occur.[1]
Risk factors include human papillomavirus (HPV), HIV/AIDS, receptive anal sex,[4] smoking, and many sexual partners.[1] Anal cancer is typically a squamous cell carcinoma.[3] Other types include adenocarcinoma, small cell carcinoma, and melanoma.[3] Diagnosis is suspected based on physical examination and confirmed by tissue biopsy.[1]
Prevention includes avoiding risk factors and HPV vaccination.[6] Standard treatment may include radiation therapy, chemotherapy, and surgery.[1] About 8,300 people are diagnosed a year in the United States, representing about 0.5% of new cancers.[2] Onset is typically after the age of 45.[2] Women are affected more often than men.[2] The number of cases has increased since the 1990s.[3] The five-year survival rate in the United States is 68%.[2]
Signs and symptoms
[edit]
Symptoms of anal cancer can include pain or pressure in the anus or rectum, a change in bowel habits, a lump near the anus, rectal bleeding, itching or discharge. Bleeding may be severe.[7][8]
Risk factors
[edit]- Human papillomavirus: Examination of squamous cell carcinoma tumor tissues from patients in Denmark and Sweden showed a high proportion of anal cancers to be positive for the types of HPV that are also associated with high risk of cervical cancer.[9] In another study done, high-risk types of HPV, notably HPV-16, were detected in 84 percent of anal cancer specimens examined.[10] Based on the study in Denmark and Sweden, Parkin estimated that 90% of anal cancers are attributable to HPV.[11]
- Sexual activity: Due to increased risk of exposure to HPV, having multiple sex partners and receptive anal intercourse greatly increases the likelihood of developing anal cancer, for men and women.[12][13][14]
- Smoking: Current smokers are several times more likely to develop anal cancer compared with nonsmokers.[14] Epidemiologist Janet Daling, Ph.D., a member of Fred Hutchinson's Public Health Sciences Division, and her team found that smoking appears to play a significant role in anal-cancer development that is independent of other behavioral risk factors, such as sexual activity. More than half of the anal-cancer patients studied were current smokers at the time of diagnosis, as compared to a smoking rate of about 23 percent among the controls. "Current smoking is a very important promoter of the disease," said Daling. "There's a fourfold increase in risk if you're a current smoker, regardless of whether you're male or female." They explained that the mechanism behind smoking and anal-cancer development is unknown, but researchers speculate that smoking interferes with a process called apoptosis, or programmed cell death, which helps rid the body of abnormal cells that could turn cancerous. Another possibility is that smoking suppresses the immune system, which can decrease the body's ability to clear persistent infection or abnormal cells.[12]
- Immunosuppression, which is often associated with HIV infection.[14]
- A history of cervical, vaginal or vulval cancers[15]
- Cloacogenic. Cloacogenic carcinoma is a rare tumor of the anorectal region originating from a persistent remnant of the cloacal membrane of the embryo. The tumor accounts for 2–3% of anorectal carcinomas and occurs more than twice as often in women.[16]
Pathology
[edit]Most anal cancers are squamous cell carcinomas (epidermoid carcinomas), that arises near the squamocolumnar junction. It may be keratinizing (basaloid) or non-keratinizing (cloacogenic).[17]
Other types of anal cancer are adenocarcinoma, lymphoma, sarcoma or melanoma.
-
Stage 1 anal cancer
-
Stage 2 anal cancer
-
Stage 3 anal cancer
-
Stage 4 anal cancer
Staging
[edit]Pathologic TNM staging of anal carcinomas:[18][19]
Primary tumor (pT)
- "TX: Primary tumor not assessed"
- "T0: No evidence of primary tumor"
- "Tis: High grade squamous intraepithelial lesion"
- "T1: Tumor ≤ 2 cm"
- "T2: Tumor > 2 cm but ≤ 5 cm"
- "T3: Tumor > 5 cm"
- "T4: Tumor of any size invading adjacent organ(s), such as the vagina, urethra or bladder"
Regional lymph nodes (pN)
- "NX: Regional lymph nodes cannot be assessed"
- "N0: No regional lymph node metastasis"
- "N1: Metastasis in inguinal, mesorectal, internal iliac or external iliac nodes"
- "N1a: Metastasis in inguinal, mesorectal or internal iliac nodes"
- "N1b: Metastasis in external iliac lymph nodes"
- "N1c: Metastasis in external iliac with any N1a nodes"
Distant metastasis (pM)
- "M0: No distant metastasis"
- "M1: Distant metastasis"
Prevention
[edit]Since many, if not most, anal cancers derive from HPV infections, and since the HPV vaccine before exposure to HPV prevents infection by some strains of the virus and has been shown to reduce the incidence of potentially precancerous lesions,[20] scientists surmise that HPV vaccination may reduce the incidence of anal cancer.[21] The efficacy of the vaccine against HPV types 16 and 18 in naive women ≤26 years old has been shown to be between 91-100% but is lower when individuals are vaccinated irrespective of baseline HPV infection at 76%.[22]
In 2010, Gardasil was approved in the US to prevent anal cancer and pre-cancerous lesions in males and females aged 9 to 26 years. The vaccine has been used before to help prevent cervical, vulvar, and vaginal cancer, and associated lesions caused by HPV types 6, 11, 16, and 18 in women.[23]
Screening
[edit]As the incidence of anal cancer has increased in recent years, screening and early detection of anal intraepithelial neoplasia (AIN) has become a necessity in patients at risk. This screening detects premalignant lesions, which are highly prevalents, and improves the staging of lesions after treatment.[24]
Anal Pap smears similar to those used in cervical cancer screening have been studied for early detection of anal cancer in high-risk individuals.[25] In 2011, an HIV clinic implemented a program to enhance access to anal cancer screening for HIV-positive men. Nurse practitioners perform anal Papanicolaou screening, and men with abnormal results receive further evaluation with high-resolution anoscopy. The program has helped identify many precancerous growths, allowing them to be safely removed.[26] A similar study was performed in women with a history of cervical cancer or high-grade cervical intraepithelial neoplasia. More than 30% had abnormal anal Pap smears and one third of those already had anal intraepithelial neoplasia.[27]
Treatment
[edit]Localised disease
[edit]Localised disease (carcinoma-in-situ) and the precursor condition, anal intraepithelial neoplasia (anal dysplasia or AIN) can be ablated with minimally invasive methods such as infrared photocoagulation.[28]
Previously, anal cancer was treated with surgery, and in early-stage disease (i.e., localised cancer of the anus without metastasis to the inguinal lymph nodes), surgery is often curative. The difficulty with surgery has been the necessity of removing the internal and external anal sphincter, with concomitant fecal incontinence. For this reason, many patients with anal cancer have required permanent colostomies.[17]
Current gold-standard therapy is the combination of chemotherapy and radiation treatment to reduce the necessity of debilitating surgery.[29] This "combined modality" approach has led to the increased preservation of an intact anal sphincter, and therefore improved quality of life after definitive treatment. Survival and cure rates are excellent, and many patients are left with a functional sphincter. Some patients have fecal incontinence after combined chemotherapy and radiation. Biopsies to document disease regression after chemotherapy and radiation were commonly advised, but are not as frequent any longer. Current chemotherapy consists of continuous infusion 5-FU over four days with bolus mitomycin given concurrently with radiation. 5-FU and cisplatin are recommended for metastatic anal cancer.[30] For stages I to III anal squamous cell carcinoma, using cisplatin instead of mitomycin C (MMC) in chemoradiation therapy doesn't improve overall survival or slow down the cancer compared to the standard treatment of 5-FU plus MMC. Although cisplatin might cause fewer blood-related side effects, 5-FU combined with MMC is still the preferred treatment for nonmetastatic anal cancer.[31] A 2024 systematic review found that chemoradiation therapy (CRT) with 5-FU and Mitomycin C improves locoregional control and colostomy-free survival compared to radiation alone, though with increased acute hematologic toxicity.[32]
Metastatic or recurrent disease
[edit]10 to 20% of patients treated for anal cancer will develop distant metastatic disease following treatment.[33] Metastatic or recurrent anal cancer is difficult to treat, and usually requires chemotherapy. Radiation is also employed to palliate specific locations of disease that may be causing symptoms. Chemotherapy commonly used is similar to other squamous cell epithelial neoplasms, such as platinum analogues, anthracyclines such as doxorubicin, and antimetabolites such as 5-FU and capecitabine. JD Hainsworth developed a protocol that includes Taxol and Carboplatinum along with 5-FU.[34]
Prognosis
[edit]Median survival rates for people with distant metastases range from 8 to 34 months.[33] Surgical resection with permanent colostomies was the standard treatment until the 1970s, yielding 5-year overall survival of approximately 50%. The best overall survival rates are seen after combined radiation therapy combined with chemotherapy (5-FU + Mitomycin) in people with T2N0 and T3N0 categories of disease (5-y overall survival: 82%). The 5-year overall survival rates of patients with T4 with no involved lymph nodes, T3 with involved lymph nodes, and T4 with involved lymph nodes disease after the combined treatment is 57%, 57%, and 42%, respectively.[35][36]
Radiation therapy side effects (and occurrence rates) include acute (27%) and late (17%) dermatological toxicities, acute (14%) and late (27%) gastrointestinal toxicities,[37] and chronic pelvic radiation disease (1-10%), e.g., irreversible lumbosacral plexopathy.[38]
Epidemiology
[edit]Worldwide in 2002 there were an estimated 30,400 new cases of anal cancer.[11] With approximately equal fractions in the developing (15,900) and developed (14,500) countries.[11] An estimated 90% (27,400) were attributable to human papillomavirus (HPV).[11]
United States
[edit]In 2014 about 7,060 new cases of anal cancer were diagnosed in the United States (4,430 in women and 2,630 in men).[39] It is typically found in adults, average age early 60s.[39] In 2019, an estimated 8,300 adults will be diagnosed with anal cancer.[40]
In the United States, an estimated 800 to 900 people die of anal cancer annually.[39]
United Kingdom
[edit]Anal cancer accounts for less than 1% of all cancer cases and deaths in the United Kingdom. Around 1,200 people were diagnosed with the disease in 2011, and around 310 people died in 2012.[41]
References
[edit]- ^ a b c d e f g h i j k "Anal Cancer Treatment". National Cancer Institute. 2018. Retrieved 30 May 2019.
- ^ a b c d e f g h "Cancer of the Anus, Anal Canal, and Anorectum—Cancer Stat Facts". SEER. Retrieved 30 May 2019.
- ^ a b c d Nelson, VM; Benson AB, 3rd (January 2017). "Epidemiology of Anal Canal Cancer". Surgical Oncology Clinics of North America. 26 (1): 9–15. doi:10.1016/j.soc.2016.07.001. PMID 27889039.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ a b Daling, Janet; et al. (1987). "Sexual Practices, Sexually Transmitted Diseases, and the Incidence of Anal Cancer". The New England Journal of Medicine. 317 (16): 973–977. doi:10.1056/NEJM198710153171601. PMID 2821396.
- ^ Garden, O. James; Bradbury, Andrew W.; Forsythe, John L. R.; Parks, Rowan W. (2012). Principles and Practice of Surgery E-Book. Elsevier Health Sciences. p. 272. ISBN 9780702051166.
- ^ a b "Anal Cancer Prevention". National Cancer Institute. 14 February 2014. Retrieved 30 May 2019.
- ^ National Cancer Institute. Anal Cancer Treatment (PDQ) Patient Version. Archived July 14, 2009, at the Wayback Machine 13 June 2008. Accessed 26 June 2009.
- ^ Stanley, Margaret A; Winder, David M; Sterling, Jane C; Goon, Peter KC (2012). "HPV infection, anal intra-epithelial neoplasia (AIN) and anal cancer: current issues". BMC Cancer. 12 (1): 398. doi:10.1186/1471-2407-12-398. ISSN 1471-2407. PMC 3488563. PMID 22958276.
- ^ Frisch M (August 2002). "On the etiology of anal squamous carcinoma". Danish Medical Bulletin. 49 (3): 194–209. PMID 12238281.
- ^ Frisch M, Glimelius B, van den Brule AJ, et al. (November 1997). "Sexually transmitted infection as a cause of anal cancer". N. Engl. J. Med. 337 (19): 1350–58. doi:10.1056/NEJM199711063371904. PMID 9358129.
- ^ a b c d Parkin DM (2006). "The global health burden of infection-associated cancers in the year 2002". Int. J. Cancer. 118 (12): 3030–44. doi:10.1002/ijc.21731. PMID 16404738. S2CID 10042384.
- ^ a b "Fred Hutchinson Cancer Research Center, Changing Trends in Sexual Behavior May Explain Rising Incidence of Anal Cancer Among American Men and Women". Fred Hutchinson Cancer Research Center (fhcrc.org). 2004-07-06. Retrieved 2010-04-21.
- ^ "STD Facts – HPV and Men". Archived from the original on 14 September 2007. Retrieved 2007-08-17.
- ^ a b c "Anal Cancer". American Cancer Society. Archived from the original on 2014-12-22. Retrieved 2014-12-22.
- ^ Saleem AM, Paulus JK, Shapter AP, Baxter NN, Roberts PL, Ricciardi R (2011). "Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm". Obstetrics and Gynecology. 117 (3): 643–49. doi:10.1097/AOG.0b013e31820bfb16. PMID 21343768. S2CID 43339714.
- ^ Sink JD, Kramer SA, Copeland DD, Seigler HF (1978). "Cloacogenic carcinoma". Annals of Surgery. 188 (1): 53–59. doi:10.1097/00000658-197807000-00009. PMC 1396653. PMID 666378.
- ^ a b "Anal Cancer". The Lecturio Medical Concept Library. Retrieved 28 June 2021.
- ^ American Joint Committee on Cancer, AJCC Cancer Staging Form Supplement, AJCC Cancer Staging Manual, Eighth Edition, Last updated 05 June 2018.
- ^ MB Amin, SB Edge, FL Greene, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017.
- ^ ""Gardasil, Merck's Cervical Cancer Vaccine, Demonstrated Efficacy in Preventing HPV-Related Disease in Males in Phase III Study: Pivotal Study Evaluating Efficacy of Gardasil in Males in Preventing HPV 6, 11, 16 and 18-Related External Genital Lesions"". Merck Research and Development News. (www.merck.com). Archived from the original on 15 December 2008. Retrieved 2008-11-15.
- ^ Tuller, David (2007-01-31). "HPV vaccine may help to prevent anal cancer". International Herald Tribune. Retrieved 2014-12-22.
- ^ Kamolratanakul S, Pitisuttithum P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines. 2021;9(12).
- ^ US approves anal cancer vaccine http://www.channelnewsasia.com/stories/health/view/1100843/1/.html Archived 2011-01-01 at the Wayback Machine
- ^ Fernández Isart, Myriam; Serra Esteban, Julia; Segura Sampedro, Juan José; Amengual Antich, Isabel; Martínez Ortega, Marco Antonio; Forteza Valades, Ana; Riera Jaume, Melchor; González Argente, Francesc Xavier (2022-03-14). "Anal intraepithelial neoplasia screening in patients with human immunodeficiency virus infection". Revista Espanola de Enfermedades Digestivas. 114 (12): 713–718. doi:10.17235/reed.2022.8489/2021. ISSN 1130-0108. PMID 35285660. S2CID 247341037.
- ^ Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H (2006). "Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review". Clin. Infect. Dis. 43 (2): 223–33. doi:10.1086/505219. PMID 16779751. S2CID 8447335.
- ^ "Hospital HIV Clinic Offers Convenient, Proactive Screening for Anal Cancer, Enabling Identification and Treatment of Precancerous Lesions". Agency for Healthcare Research and Quality. 2013-04-10. Retrieved 2013-05-10.
- ^ Wohlmuth, Christoph; Ghorab, Zeina; Shier, Michael; Tinmouth, Jill; Salit, Irving E.; Covens, Allan; Zhang, Liying; Vicus, Danielle (2020-10-01). "Cytology-based screening for anal intraepithelial neoplasia in women with a history of cervical intraepithelial neoplasia or cancer". Cancer Cytopathology. 129 (2): 140–147. doi:10.1002/cncy.22360. ISSN 1934-6638. PMID 33002327. S2CID 222168316.
- ^ Goldstone SE, Kawalek AZ, Huyett JW (2005). "Infrared coagulator: a useful tool for treating anal squamous intraepithelial lesions". Diseases of the Colon and Rectum. 48 (5): 1042–54. doi:10.1007/s10350-004-0889-0. PMID 15868241. S2CID 25694842.
- ^ National Comprehensive Cancer Network. "NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma. V 1.2013" (PDF).
- ^ Ghosn M, Kourie HR, Abdayem P, Antoun J, Nasr D (2015). "Anal cancer treatment: current status and future perspectives". World J. Gastroenterol. 21 (8): 2294–302. doi:10.3748/wjg.v21.i8.2294. PMC 4342904. PMID 25741135.
- ^ "Treatment of Stages I–III Squamous Cell Anal Cancer: A Systematic Review". Agency for Healthcare Research and Quality. doi:10.23970/AHRQEPCCER273. Retrieved 2024-10-18.
- ^ Troester, Alexander; Parikh, Romil; Southwell, Bronwyn; Ester, Elizabeth; Sultan, Shahnaz; Greeno, Edward; Arsoniadis, Elliot; Church, Timothy R; Wilt, Timothy; Butler, Mary; Goffredo, Paolo (2024-08-20). "Treatment of stage I-III squamous cell anal cancer: a comparative effectiveness systematic review". JNCI: Journal of the National Cancer Institute. doi:10.1093/jnci/djae195. ISSN 0027-8874.
- ^ a b Dewdney A, Rao S (2012). "Metastatic squamous cell carcinoma of the anus: time for a shift in the treatment paradigm?". ISRN Oncology. 2012: 1–6. doi:10.5402/2012/756591. PMC 3352602. PMID 22619735.
- ^ "Fluorouracil". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ Gunderson et al., Anal Carcinoma: Impact of TN Category of Disease on Survival, Disease Relapse, and Colostomy Failure in US Gastrointestinal Intergroup RTOG 98-11 Phase 3 Trial. Int J Radiation Oncol Biol Phys, 2013, Vol. 87, No. 4, pp. 638–645.
- ^ Suzanne Russo, et al., Executive Summary of the American Radium Society Appropriate Use Criteria for Treatment of Anal Cancer. Int J Radiat Oncol Biol Phys. 2019 Nov 1;105(3):591–605
- ^ Troester, Alexander; Parikh, Romil; Southwell, Bronwyn; Ester, Elizabeth; Sultan, Shahnaz; Greeno, Edward; Arsoniadis, Elliot; Church, Timothy R; Wilt, Timothy; Butler, Mary; Goffredo, Paolo (2024-08-20). "Treatment of stage I-III squamous cell anal cancer: a comparative effectiveness systematic review". JNCI: Journal of the National Cancer Institute. doi:10.1093/jnci/djae195. ISSN 0027-8874.
- ^ Huh, Jung Wook; Tanksley, Jarred; Chino, Junzo; Willett, Christopher G.; Dewhirst, Mark W. (July 1, 2020). "Long-term Consequences of Pelvic Irradiation: Toxicities, Challenges, and Therapeutic Opportunities with Pharmacologic Mitigators". Clinical Cancer Research. 26 (13): 3079–3090. doi:10.1158/1078-0432.CCR-19-2744. ISSN 1078-0432. Retrieved 24 October 2024.
- ^ a b c "Detailed Guide: Anal Cancer What Are the Key Statistics About Anal Cancer?". Archived from the original on 2016-12-03. Retrieved 2008-11-18.
- ^ "Anal Cancer: Statistics". Cancer.net. 25 June 2012. Retrieved November 20, 2019.
- ^ "Anal Cancer Statistics". Cancer Research UK. Retrieved 27 October 2014.