X-ray

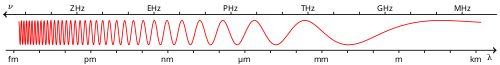
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ranging from 10 nanometers to 10 picometers, corresponding to frequencies in the range of 30 petahertz to 30 exahertz (3×1016 Hz to 3×1019 Hz) and photon energies in the range of 100 eV to 100 keV, respectively.[1]
X-rays were discovered in 1895 by the German scientist Wilhelm Conrad Röntgen,[2] who named it X-radiation to signify an unknown type of radiation.[3]
X-rays can penetrate many solid substances such as construction materials and living tissue,[4] so X-ray radiography is widely used in medical diagnostics (e.g., checking for broken bones) and material science (e.g., identification of some chemical elements and detecting weak points in construction materials).[5] However X-rays are ionizing radiation, and exposure to high intensities can be hazardous to health, causing damage to DNA, cancer, and at high dosages, burns and radiation sickness. Their generation and use is strictly controlled by public health authorities.
History
[edit]Pre-Röntgen observations and research
[edit]
X-rays were originally noticed in science as a type of unidentified radiation emanating from discharge tubes by experimenters investigating cathode rays produced by such tubes, which are energetic electron beams that were first observed in 1869. Early researchers noticed effects that were attributable to them in many of the early Crookes tubes (invented around 1875). Crookes tubes created free electrons by ionization of the residual air in the tube by a high DC voltage of anywhere between a few kilovolts and 100 kV. This voltage accelerated the electrons coming from the cathode to a high enough velocity that they created X-rays when they struck the anode or the glass wall of the tube.[6]
The earliest experimenter thought to have (unknowingly) produced X-rays was William Morgan. In 1785, he presented a paper to the Royal Society of London describing the effects of passing electrical currents through a partially evacuated glass tube, producing a glow created by X-rays.[7][8] This work was further explored by Humphry Davy and his assistant Michael Faraday.
Starting in 1888, Philipp Lenard conducted experiments to see whether cathode rays could pass out of the Crookes tube into the air. He built a Crookes tube with a "window" at the end made of thin aluminium, facing the cathode so the cathode rays would strike it (later called a "Lenard tube"). He found that something came through, that would expose photographic plates and cause fluorescence. He measured the penetrating power of these rays through various materials. It has been suggested that at least some of these "Lenard rays" were actually X-rays.[9]
Helmholtz formulated mathematical equations for X-rays. He postulated a dispersion theory before Röntgen made his discovery and announcement. He based it on the electromagnetic theory of light.[10][full citation needed] However, he did not work with actual X-rays.
In early 1890, photographer William Jennings and associate professor of the University of Pennsylvania Arthur W. Goodspeed were making photographs of coins with electric sparks. On 22nd February after the end of their experiments two coins were left on a stack of photographic plates before Goodspeed demonstrated to Jennings the operation of Crookes tubes. While developing the plates, Jennings noticed disks of unknown origin on some of the plates, but nobody could explain them, and they moved on. Only in 1896 they realized that they accidentally made an X-ray photograph (they didn't claim a discovery).[11]
Also in 1890, Roentgen's assistant Ludwig Zehnder noticed a flash of light from a fluorescent screen immediately before the covered tube he was switching on punctured.[12]
When Stanford University physics professor Fernando Sanford conducted his "electric photography" experiments in 1891-1893 by photographing coins in the light of electric sparks,[13] like Jennings and Goodspeed, he may have unknowingly generated and detected X-rays. His letter of 6 January 1893 to the Physical Review was duly published[13] and an article entitled Without Lens or Light, Photographs Taken With Plate and Object in Darkness appeared in the San Francisco Examiner.[14]
In 1894, Nikola Tesla noticed damaged film in his lab that seemed to be associated with Crookes tube experiments and began investigating this invisible, radiant energy.[15][16] After Röntgen identified the X-ray, Tesla began making X-ray images of his own using high voltages and tubes of his own design,[17] as well as Crookes tubes.
Discovery by Röntgen
[edit]
On 8 November 1895, German physics professor Wilhelm Röntgen stumbled on X-rays while experimenting with Lenard tubes and Crookes tubes and began studying them. He wrote an initial report "On a new kind of ray: A preliminary communication" and on 28 December 1895, submitted it to Würzburg's Physical-Medical Society journal.[18] This was the first paper written on X-rays. Röntgen referred to the radiation as "X", to indicate that it was an unknown type of radiation. Some early texts refer to them as Chi-rays, having interpreted "X" as the uppercase Greek letter Chi, Χ.[19][20][21]
There are conflicting accounts of his discovery because Röntgen had his lab notes burned after his death, but this is a likely reconstruction by his biographers:[22][23] Röntgen was investigating cathode rays from a Crookes tube which he had wrapped in black cardboard so that the visible light from the tube would not interfere, using a fluorescent screen painted with barium platinocyanide. He noticed a faint green glow from the screen, about 1 meter (3.3 ft) away. Röntgen realized some invisible rays coming from the tube were passing through the cardboard to make the screen glow. He found they could also pass through books and papers on his desk. Röntgen threw himself into investigating these unknown rays systematically. Two months after his initial discovery, he published his paper.[24]
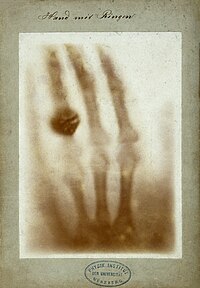
Röntgen discovered their medical use when he made a picture of his wife's hand on a photographic plate formed due to X-rays. The photograph of his wife's hand was the first photograph of a human body part using X-rays. When she saw the picture, she said "I have seen my death."[27]
The discovery of X-rays generated significant interest. Röntgen's biographer Otto Glasser estimated that, in 1896 alone, as many as 49 essays and 1044 articles about the new rays were published.[28] This was probably a conservative estimate, if one considers that nearly every paper around the world extensively reported about the new discovery, with a magazine such as Science dedicating as many as 23 articles to it in that year alone.[29] Sensationalist reactions to the new discovery included publications linking the new kind of rays to occult and paranormal theories, such as telepathy.[30][31]
The name X-rays stuck, although (over Röntgen's great objections) many of his colleagues suggested calling them Röntgen rays. They are still referred to as such in many languages, including German, Hungarian, Ukrainian, Danish, Polish, Czech, Bulgarian, Swedish, Finnish, Portuguese, Estonian, Slovak, Slovenian, Turkish, Russian, Latvian, Lithuanian, Albanian, Japanese, Dutch, Georgian, Hebrew, Icelandic, and Norwegian.[original research?]
Röntgen received the first Nobel Prize in Physics for his discovery.[32]
Advances in radiology
[edit]

Röntgen immediately noticed X-rays could have medical applications. Along with his 28 December Physical-Medical Society submission, he sent a letter to physicians he knew around Europe (1 January 1896).[33] News (and the creation of "shadowgrams") spread rapidly with Scottish electrical engineer Alan Archibald Campbell-Swinton being the first after Röntgen to create an X-ray photograph (of a hand). Through February, there were 46 experimenters taking up the technique in North America alone.[33]
The first use of X-rays under clinical conditions was by John Hall-Edwards in Birmingham, England on 11 January 1896, when he radiographed a needle stuck in the hand of an associate. On 14 February 1896, Hall-Edwards was also the first to use X-rays in a surgical operation.[34]

In early 1896, several weeks after Röntgen's discovery, Ivan Romanovich Tarkhanov irradiated frogs and insects with X-rays, concluding that the rays "not only photograph, but also affect the living function".[35] At around the same time, the zoological illustrator James Green began to use X-rays to examine fragile specimens. George Albert Boulenger first mentioned this work in a paper he delivered before the Zoological Society of London in May 1896. The book Sciagraphs of British Batrachians and Reptiles (sciagraph is an obsolete name for an X-ray photograph), by Green and James H. Gardiner, with a foreword by Boulenger, was published in 1897.[36][37]
The first medical X-ray made in the United States was obtained using a discharge tube of Puluj's design. In January 1896, on reading of Röntgen's discovery, Frank Austin of Dartmouth College tested all of the discharge tubes in the physics laboratory and found that only the Puluj tube produced X-rays. This was a result of Puluj's inclusion of an oblique "target" of mica, used for holding samples of fluorescent material, within the tube. On 3 February 1896, Gilman Frost, professor of medicine at the college, and his brother Edwin Frost, professor of physics, exposed the wrist of Eddie McCarthy, whom Gilman had treated some weeks earlier for a fracture, to the X-rays and collected the resulting image of the broken bone on gelatin photographic plates obtained from Howard Langill, a local photographer also interested in Röntgen's work.[38]

Many experimenters, including Röntgen himself in his original experiments, came up with methods to view X-ray images "live" using some form of luminescent screen.[33] Röntgen used a screen coated with barium platinocyanide. On 5 February 1896, live imaging devices were developed by both Italian scientist Enrico Salvioni (his "cryptoscope") and William Francis Magie of Princeton University (his "Skiascope"), both using barium platinocyanide. American inventor Thomas Edison started research soon after Röntgen's discovery and investigated materials' ability to fluoresce when exposed to X-rays, finding that calcium tungstate was the most effective substance. In May 1896, he developed the first mass-produced live imaging device, his "Vitascope", later called the fluoroscope, which became the standard for medical X-ray examinations.[33] Edison dropped X-ray research around 1903, before the death of Clarence Madison Dally, one of his glassblowers. Dally had a habit of testing X-ray tubes on his own hands, developing a cancer in them so tenacious that both arms were amputated in a futile attempt to save his life; in 1904, he became the first known death attributed to X-ray exposure.[33] During the time the fluoroscope was being developed, Serbian American physicist Mihajlo Pupin, using a calcium tungstate screen developed by Edison, found that using a fluorescent screen decreased the exposure time it took to create an X-ray for medical imaging from an hour to a few minutes.[39][33]
In 1901, U.S. President William McKinley was shot twice in an assassination attempt while attending the Pan American Exposition in Buffalo, New York. While one bullet only grazed his sternum, another had lodged somewhere deep inside his abdomen and could not be found. A worried McKinley aide sent word to inventor Thomas Edison to rush an X-ray machine to Buffalo to find the stray bullet. It arrived but was not used. While the shooting itself had not been lethal, gangrene had developed along the path of the bullet, and McKinley died of septic shock due to bacterial infection six days later.[40]
Hazards discovered
[edit]With the widespread experimentation with X‑rays after their discovery in 1895 by scientists, physicians, and inventors came many stories of burns, hair loss, and worse in technical journals of the time. In February 1896, Professor John Daniel and William Lofland Dudley of Vanderbilt University reported hair loss after Dudley was X-rayed. A child who had been shot in the head was brought to the Vanderbilt laboratory in 1896. Before trying to find the bullet, an experiment was attempted, for which Dudley "with his characteristic devotion to science"[41][42][43] volunteered. Daniel reported that 21 days after taking a picture of Dudley's skull (with an exposure time of one hour), he noticed a bald spot 5 centimeters (2 in) in diameter on the part of his head nearest the X-ray tube: "A plate holder with the plates towards the side of the skull was fastened and a coin placed between the skull and the head. The tube was fastened at the other side at a distance of one-half-inch [1.3 cm] from the hair."[44] Beyond burns, hair loss, and cancer, X-rays can be linked to infertility in males based on the amount of radiation used.
In August 1896, H. D. Hawks, a graduate of Columbia College, suffered severe hand and chest burns from an X-ray demonstration. It was reported in Electrical Review and led to many other reports of problems associated with X-rays being sent in to the publication.[45] Many experimenters including Elihu Thomson at Edison's lab, William J. Morton, and Nikola Tesla also reported burns. Elihu Thomson deliberately exposed a finger to an X-ray tube over a period of time and suffered pain, swelling, and blistering.[46] Other effects were sometimes blamed for the damage including ultraviolet rays and (according to Tesla) ozone.[15] Many physicians claimed there were no effects from X-ray exposure at all.[46] On 3 August 1905, in San Francisco, California, Elizabeth Fleischman, an American X-ray pioneer, died from complications as a result of her work with X-rays.[47][48][49]
Hall-Edwards developed a cancer (then called X-ray dermatitis) sufficiently advanced by 1904 to cause him to write papers and give public addresses on the dangers of X-rays. His left arm had to be amputated at the elbow in 1908,[50][51] and four fingers on his right arm soon thereafter, leaving only a thumb. He died of cancer in 1926. His left hand is kept at Birmingham University.
20th century and beyond
[edit]
The many applications of X-rays immediately generated enormous interest. Workshops began making specialized versions of Crookes tubes for generating X-rays and these first-generation cold cathode or Crookes X-ray tubes were used until about 1920.
A typical early 20th-century medical X-ray system consisted of a Ruhmkorff coil connected to a cold cathode Crookes X-ray tube. A spark gap was typically connected to the high voltage side in parallel to the tube and used for diagnostic purposes.[52] The spark gap allowed detecting the polarity of the sparks, measuring voltage by the length of the sparks thus determining the "hardness" of the vacuum of the tube, and it provided a load in the event the X-ray tube was disconnected. To detect the hardness of the tube, the spark gap was initially opened to the widest setting. While the coil was operating, the operator reduced the gap until sparks began to appear. A tube in which the spark gap began to spark at around 6.4 centimeters (2.5 in) was considered soft (low vacuum) and suitable for thin body parts such as hands and arms. A 13-centimeter (5 in) spark indicated the tube was suitable for shoulders and knees. An 18-to-23-centimeter (7 to 9 in) spark would indicate a higher vacuum suitable for imaging the abdomen of larger individuals. Since the spark gap was connected in parallel to the tube, the spark gap had to be opened until the sparking ceased to operate the tube for imaging. Exposure time for photographic plates was around half a minute for a hand to a couple of minutes for a thorax. The plates may have a small addition of fluorescent salt to reduce exposure times.[52]
Crookes tubes were unreliable. They had to contain a small quantity of gas (invariably air) as a current will not flow in such a tube if they are fully evacuated. However, as time passed, the X-rays caused the glass to absorb the gas, causing the tube to generate "harder" X-rays until it soon stopped operating. Larger and more frequently used tubes were provided with devices for restoring the air, known as "softeners". These often took the form of a small side tube that contained a small piece of mica, a mineral that traps relatively large quantities of air within its structure. A small electrical heater heated the mica, causing it to release a small amount of air, thus restoring the tube's efficiency. However, the mica had a limited life, and the restoration process was difficult to control.
In 1904, John Ambrose Fleming invented the thermionic diode, the first kind of vacuum tube. This used a hot cathode that caused an electric current to flow in a vacuum. This idea was quickly applied to X-ray tubes, and hence heated-cathode X-ray tubes, called "Coolidge tubes", completely replaced the troublesome cold cathode tubes by about 1920.
In about 1906, the physicist Charles Barkla discovered that X-rays could be scattered by gases, and that each element had a characteristic X-ray spectrum. He won the 1917 Nobel Prize in Physics for this discovery.
In 1912, Max von Laue, Paul Knipping, and Walter Friedrich first observed the diffraction of X-rays by crystals. This discovery, along with the early work of Paul Peter Ewald, William Henry Bragg, and William Lawrence Bragg, gave birth to the field of X-ray crystallography.[53]
In 1913, Henry Moseley performed crystallography experiments with X-rays emanating from various metals and formulated Moseley's law which relates the frequency of the X-rays to the atomic number of the metal.
The Coolidge X-ray tube was invented the same year by William D. Coolidge. It made possible the continuous emissions of X-rays. Modern X-ray tubes are based on this design, often employing the use of rotating targets which allow for significantly higher heat dissipation than static targets, further allowing higher quantity X-ray output for use in high-powered applications such as rotational CT scanners.

The use of X-rays for medical purposes (which developed into the field of radiation therapy) was pioneered by Major John Hall-Edwards in Birmingham, England. Then in 1908, he had to have his left arm amputated because of the spread of X-ray dermatitis on his arm.[54]
Medical science also used the motion picture to study human physiology. In 1913, a motion picture was made in Detroit showing a hard-boiled egg inside a human stomach. This early X-ray movie was recorded at a rate of one still image every four seconds.[55] Dr Lewis Gregory Cole of New York was a pioneer of the technique, which he called "serial radiography".[56][57] In 1918, X-rays were used in association with motion picture cameras to capture the human skeleton in motion.[58][59][60] In 1920, it was used to record the movements of tongue and teeth in the study of languages by the Institute of Phonetics in England.[61]
In 1914, Marie Curie developed radiological cars to support soldiers injured in World War I. The cars would allow for rapid X-ray imaging of wounded soldiers so battlefield surgeons could quickly and more accurately operate.[62]
From the early 1920s through to the 1950s, X-ray machines were developed to assist in the fitting of shoes[63] and were sold to commercial shoe stores.[64][65][66] Concerns regarding the impact of frequent or poorly controlled use were expressed in the 1950s,[67][68] leading to the practice's eventual end that decade.[69]
The X-ray microscope was developed during the 1950s.
The Chandra X-ray Observatory, launched on 23 July 1999, has been allowing the exploration of the very violent processes in the universe that produce X-rays. Unlike visible light, which gives a relatively stable view of the universe, the X-ray universe is unstable. It features stars being torn apart by black holes, galactic collisions, and novae, and neutron stars that build up layers of plasma that then explode into space.
An X-ray laser device was proposed as part of the Reagan Administration's Strategic Defense Initiative in the 1980s, but the only test of the device (a sort of laser "blaster" or death ray, powered by a thermonuclear explosion) gave inconclusive results. For technical and political reasons, the overall project (including the X-ray laser) was defunded (though was later revived by the second Bush Administration as National Missile Defense using different technologies).
Phase-contrast X-ray imaging refers to a variety of techniques that use phase information of an X-ray beam to form the image. Due to its good sensitivity to density differences, it is especially useful for imaging soft tissues. It has become an important method for visualizing cellular and histological structures in a wide range of biological and medical studies. There are several technologies being used for X-ray phase-contrast imaging, all using different principles to convert phase variations in the X-rays emerging from an object into intensity variations.[70][71] These include propagation-based phase contrast,[72] Talbot interferometry,[71] refraction-enhanced imaging,[73] and X-ray interferometry.[74] These methods provide higher contrast compared to normal absorption-based X-ray imaging, making it possible to distinguish from each other details that have almost similar density. A disadvantage is that these methods require more sophisticated equipment, such as synchrotron or microfocus X-ray sources, X-ray optics, and high resolution X-ray detectors.
Energy ranges
[edit]
Soft and hard X-rays
[edit]X-rays with high photon energies above 5–10 keV (below 0.2–0.1 nm wavelength) are called hard X-rays, while those with lower energy (and longer wavelength) are called soft X-rays.[75] The intermediate range with photon energies of several keV is often referred to as tender X-rays. Due to their penetrating ability, hard X-rays are widely used to image the inside of objects (e.g. in medical radiography and airport security). The term X-ray is metonymically used to refer to a radiographic image produced using this method, in addition to the method itself. Since the wavelengths of hard X-rays are similar to the size of atoms, they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air; the attenuation length of 600 eV (~2 nm) X-rays in water is less than 1 micrometer.[76]
Gamma rays
[edit]There is no consensus for a definition distinguishing between X-rays and gamma rays. One common practice is to distinguish between the two types of radiation based on their source: X-rays are emitted by electrons, while gamma rays are emitted by the atomic nucleus.[77][78][79][80] This definition has several problems: other processes can also generate these high-energy photons, or sometimes the method of generation is not known. One common alternative is to distinguish X- and gamma radiation on the basis of wavelength (or, equivalently, frequency or photon energy), with radiation shorter than some arbitrary wavelength, such as 10−11 m (0.1 Å), defined as gamma radiation.[81] This criterion assigns a photon to an unambiguous category, but is only possible if wavelength is known. (Some measurement techniques do not distinguish between detected wavelengths.) However, these two definitions often coincide since the electromagnetic radiation emitted by X-ray tubes generally has a longer wavelength and lower photon energy than the radiation emitted by radioactive nuclei.[77] Occasionally, one term or the other is used in specific contexts due to historical precedent, based on measurement (detection) technique, or based on their intended use rather than their wavelength or source. Thus, gamma-rays generated for medical and industrial uses, for example radiotherapy, in the ranges of 6–20 MeV, can in this context also be referred to as X-rays.[82]
Properties
[edit]
X-ray photons carry enough energy to ionize atoms and disrupt molecular bonds. This makes it a type of ionizing radiation, and therefore harmful to living tissue. A very high radiation dose over a short period of time causes burns and radiation sickness, while lower doses can give an increased risk of radiation-induced cancer. In medical imaging, this increased cancer risk is generally greatly outweighed by the benefits of the examination. The ionizing capability of X-rays can be used in cancer treatment to kill malignant cells using radiation therapy. It is also used for material characterization using X-ray spectroscopy.
Hard X-rays can traverse relatively thick objects without being much absorbed or scattered. For this reason, X-rays are widely used to image the inside of visually opaque objects. The most often seen applications are in medical radiography and airport security scanners, but similar techniques are also important in industry (e.g. industrial radiography and industrial CT scanning) and research (e.g. small animal CT). The penetration depth varies with several orders of magnitude over the X-ray spectrum. This allows the photon energy to be adjusted for the application so as to give sufficient transmission through the object and at the same time provide good contrast in the image.
X-rays have much shorter wavelengths than visible light, which makes it possible to probe structures much smaller than can be seen using a normal microscope. This property is used in X-ray microscopy to acquire high-resolution images, and also in X-ray crystallography to determine the positions of atoms in crystals.
Interaction with matter
[edit]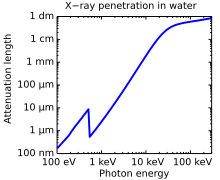
X-rays interact with matter in three main ways, through photoabsorption, Compton scattering, and Rayleigh scattering. The strength of these interactions depends on the energy of the X-rays and the elemental composition of the material, but not much on chemical properties, since the X-ray photon energy is much higher than chemical binding energies. Photoabsorption or photoelectric absorption is the dominant interaction mechanism in the soft X-ray regime and for the lower hard X-ray energies. At higher energies, Compton scattering dominates.
Photoelectric absorption
[edit]The probability of a photoelectric absorption per unit mass is approximately proportional to , where is the atomic number and is the energy of the incident photon.[83] This rule is not valid close to inner shell electron binding energies where there are abrupt changes in interaction probability, so called absorption edges. However, the general trend of high absorption coefficients and thus short penetration depths for low photon energies and high atomic numbers is very strong. For soft tissue, photoabsorption dominates up to about 26 keV photon energy where Compton scattering takes over. For higher atomic number substances, this limit is higher. The high amount of calcium () in bones, together with their high density, is what makes them show up so clearly on medical radiographs.
A photoabsorbed photon transfers all its energy to the electron with which it interacts, thus ionizing the atom to which the electron was bound and producing a photoelectron that is likely to ionize more atoms in its path. An outer electron will fill the vacant electron position and produce either a characteristic X-ray or an Auger electron. These effects can be used for elemental detection through X-ray spectroscopy or Auger electron spectroscopy.
Compton scattering
[edit]Compton scattering is the predominant interaction between X-rays and soft tissue in medical imaging.[84] Compton scattering is an inelastic scattering of the X-ray photon by an outer shell electron. Part of the energy of the photon is transferred to the scattering electron, thereby ionizing the atom and increasing the wavelength of the X-ray. The scattered photon can go in any direction, but a direction similar to the original direction is more likely, especially for high-energy X-rays. The probability for different scattering angles is described by the Klein–Nishina formula. The transferred energy can be directly obtained from the scattering angle from the conservation of energy and momentum.
Rayleigh scattering
[edit]Rayleigh scattering is the dominant elastic scattering mechanism in the X-ray regime.[85] Inelastic forward scattering gives rise to the refractive index, which for X-rays is only slightly below 1.[86]
Production
[edit]Whenever charged particles (electrons or ions) of sufficient energy hit a material, X-rays are produced.
Production by electrons
[edit]| Anode material |
Atomic number |
Photon energy [keV] | Wavelength [nm] | ||
|---|---|---|---|---|---|
| Kα1 | Kβ1 | Kα1 | Kβ1 | ||
| W | 74 | 59.3 | 67.2 | 0.0209 | 0.0184 |
| Mo | 42 | 17.5 | 19.6 | 0.0709 | 0.0632 |
| Cu | 29 | 8.05 | 8.91 | 0.154 | 0.139 |
| Ag | 47 | 22.2 | 24.9 | 0.0559 | 0.0497 |
| Ga | 31 | 9.25 | 10.26 | 0.134 | 0.121 |
| In | 49 | 24.2 | 27.3 | 0.0512 | 0.0455 |

X-rays can be generated by an X-ray tube, a vacuum tube that uses a high voltage to accelerate the electrons released by a hot cathode to a high velocity. The high velocity electrons collide with a metal target, the anode, creating the X-rays.[89] In medical X-ray tubes the target is usually tungsten or a more crack-resistant alloy of rhenium (5%) and tungsten (95%), but sometimes molybdenum for more specialized applications, such as when softer X-rays are needed as in mammography. In crystallography, a copper target is most common, with cobalt often being used when fluorescence from iron content in the sample might otherwise present a problem.
The maximum energy of the produced X-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 80 kV tube cannot create X-rays with an energy greater than 80 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
- Characteristic X-ray emission (X-ray electroluminescence): If the electron has enough energy, it can knock an orbital electron out of the inner electron shell of the target atom. After that, electrons from higher energy levels fill the vacancies, and X-ray photons are emitted. This process produces an emission spectrum of X-rays at a few discrete frequencies, sometimes referred to as spectral lines. Usually, these are transitions from the upper shells to the K shell (called K lines), to the L shell (called L lines) and so on. If the transition is from 2p to 1s, it is called Kα, while if it is from 3p to 1s it is Kβ. The frequencies of these lines depend on the material of the target and are therefore called characteristic lines. The Kα line usually has greater intensity than the Kβ one and is more desirable in diffraction experiments. Thus the Kβ line is filtered out by a filter. The filter is usually made of a metal having one proton less than the anode material (e.g. Ni filter for Cu anode or Nb filter for Mo anode).
- Bremsstrahlung: This is radiation given off by the electrons as they are scattered by the strong electric field near the nuclei. These X-rays have a continuous spectrum. The frequency of Bremsstrahlung is limited by the energy of incident electrons.
So, the resulting output of a tube consists of a continuous Bremsstrahlung spectrum falling off to zero at the tube voltage, plus several spikes at the characteristic lines. The voltages used in diagnostic X-ray tubes range from roughly 20 kV to 150 kV and thus the highest energies of the X-ray photons range from roughly 20 keV to 150 keV.[90]
Both of these X-ray production processes are inefficient, with only about one percent of the electrical energy used by the tube converted into X-rays, and thus most of the electric power consumed by the tube is released as waste heat. When producing a usable flux of X-rays, the X-ray tube must be designed to dissipate the excess heat.
A specialized source of X-rays which is becoming widely used in research is synchrotron radiation, which is generated by particle accelerators. Its unique features are X-ray outputs many orders of magnitude greater than those of X-ray tubes, wide X-ray spectra, excellent collimation, and linear polarization.[91]
Short nanosecond bursts of X-rays peaking at 15 keV in energy may be reliably produced by peeling pressure-sensitive adhesive tape from its backing in a moderate vacuum. This is likely to be the result of recombination of electrical charges produced by triboelectric charging. The intensity of X-ray triboluminescence is sufficient for it to be used as a source for X-ray imaging.[92]
Production by fast positive ions
[edit]X-rays can also be produced by fast protons or other positive ions. The proton-induced X-ray emission or particle-induced X-ray emission is widely used as an analytical procedure. For high energies, the production cross section is proportional to Z12Z2−4, where Z1 refers to the atomic number of the ion, Z2 refers to that of the target atom.[93] An overview of these cross sections is given in the same reference.
Production in lightning and laboratory discharges
[edit]X-rays are also produced in lightning accompanying terrestrial gamma-ray flashes. The underlying mechanism is the acceleration of electrons in lightning related electric fields and the subsequent production of photons through Bremsstrahlung.[94] This produces photons with energies of some few keV and several tens of MeV.[95] In laboratory discharges with a gap size of approximately 1 meter length and a peak voltage of 1 MV, X-rays with a characteristic energy of 160 keV are observed.[96] A possible explanation is the encounter of two streamers and the production of high-energy run-away electrons;[97] however, microscopic simulations have shown that the duration of electric field enhancement between two streamers is too short to produce a significant number of run-away electrons.[98] Recently, it has been proposed that air perturbations in the vicinity of streamers can facilitate the production of run-away electrons and hence of X-rays from discharges.[99][100]
Detectors
[edit]X-ray detectors vary in shape and function depending on their purpose. Imaging detectors such as those used for radiography were originally based on photographic plates and later photographic film, but are now mostly replaced by various digital detector types such as image plates and flat panel detectors. For radiation protection direct exposure hazard is often evaluated using ionization chambers, while dosimeters are used to measure the radiation dose the person has been exposed to. X-ray spectra can be measured either by energy dispersive or wavelength dispersive spectrometers. For X-ray diffraction applications, such as X-ray crystallography, hybrid photon counting detectors are widely used.[101]
Medical uses
[edit]This section needs additional citations for verification. (November 2017) |


Since Röntgen's discovery that X-rays can identify bone structures, X-rays have been used for medical imaging.[102] The first medical use was less than a month after his paper on the subject.[38] Up to 2010, five billion medical imaging examinations had been conducted worldwide.[103] Radiation exposure from medical imaging in 2006 made up about 50% of total ionizing radiation exposure in the United States.[104]
Projectional radiographs
[edit]
Projectional radiography is the practice of producing two-dimensional images using X-ray radiation. Bones contain a high concentration of calcium, which, due to its relatively high atomic number, absorbs X-rays efficiently. This reduces the amount of X-rays reaching the detector in the shadow of the bones, making them clearly visible on the radiograph. The lungs and trapped gas also show up clearly because of lower absorption compared to tissue, while differences between tissue types are harder to see.[105]
Projectional radiographs are useful in the detection of pathology of the skeletal system as well as for detecting some disease processes in soft tissue. Some notable examples are the very common chest X-ray, which can be used to identify lung diseases such as pneumonia, lung cancer, or pulmonary edema, and the abdominal x-ray, which can detect bowel (or intestinal) obstruction, free air (from visceral perforations), and free fluid (in ascites). X-rays may also be used to detect pathology such as gallstones (which are rarely radiopaque) or kidney stones which are often (but not always) visible. Traditional plain X-rays are less useful in the imaging of soft tissues such as the brain or muscle. One area where projectional radiographs are used extensively is in evaluating how an orthopedic implant, such as a knee, hip or shoulder replacement, is situated in the body with respect to the surrounding bone. This can be assessed in two dimensions from plain radiographs, or it can be assessed in three dimensions if a technique called '2D to 3D registration' is used. This technique purportedly negates projection errors associated with evaluating implant position from plain radiographs.[106]
Dental radiography is commonly used in the diagnoses of common oral problems, such as cavities.
In medical diagnostic applications, the low energy (soft) X-rays are unwanted, since they are totally absorbed by the body, increasing the radiation dose without contributing to the image. Hence, a thin metal sheet, often of aluminium, called an X-ray filter, is usually placed over the window of the X-ray tube, absorbing the low energy part in the spectrum. This is called hardening the beam since it shifts the center of the spectrum towards higher energy (or harder) X-rays.
To generate an image of the cardiovascular system, including the arteries and veins (angiography) an initial image is taken of the anatomical region of interest. A second image is then taken of the same region after an iodinated contrast agent has been injected into the blood vessels within this area. These two images are then digitally subtracted, leaving an image of only the iodinated contrast outlining the blood vessels. The radiologist or surgeon then compares the image obtained to normal anatomical images to determine whether there is any damage or blockage of the vessel.
Computed tomography
[edit]
Computed tomography (CT scanning) is a medical imaging modality where tomographic images or slices of specific areas of the body are obtained from a large series of two-dimensional X-ray images taken in different directions.[107] These cross-sectional images can be combined into a three-dimensional image of the inside of the body.[108] CT scans are a quicker and more cost effective imaging modality that can be used for diagnostic and therapeutic purposes in various medical disciplines.[108]
Fluoroscopy
[edit]Fluoroscopy is an imaging technique commonly used by physicians or radiation therapists to obtain real-time moving images of the internal structures of a patient through the use of a fluoroscope.[109] In its simplest form, a fluoroscope consists of an X-ray source and a fluorescent screen, between which a patient is placed. However, modern fluoroscopes couple the screen to an X-ray image intensifier and CCD video camera allowing the images to be recorded and played on a monitor. This method may use a contrast material. Examples include cardiac catheterization (to examine for coronary artery blockages), embolization procedures (to stop bleeding during hemorrhoidal artery embolization), and barium swallow (to examine for esophageal disorders and swallowing disorders). As of recent, modern fluoroscopy utilizes short bursts of x-rays, rather than a continuous beam, to effectively lower radiation exposure for both the patient and operator.[109]
Radiotherapy
[edit]The use of X-rays as a treatment is known as radiation therapy and is largely used for the management (including palliation) of cancer; it requires higher radiation doses than those received for imaging alone. X-rays beams are used for treating skin cancers using lower energy X-ray beams while higher energy beams are used for treating cancers within the body such as brain, lung, prostate, and breast.[110][111]
Adverse effects
[edit]The examples and perspective in this section deal primarily with the United States and do not represent a worldwide view of the subject. (September 2024) |
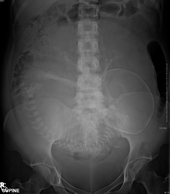
X-rays are a form of ionizing radiation, and are classified as a carcinogen by both the World Health Organization's International Agency for Research on Cancer and the U.S. government.[103][112] Diagnostic X-rays (primarily from CT scans due to the large dose used) increase the risk of developmental problems and cancer in those exposed.[113][114][115] It is estimated that 0.4% of current cancers in the United States are due to computed tomography (CT scans) performed in the past and that this may increase to as high as 1.5–2% with 2007 rates of CT usage.[116]
Experimental and epidemiological data currently do not support the proposition that there is a threshold dose of radiation below which there is no increased risk of cancer.[117] However, this is under increasing doubt.[118] Cancer risk can start at the exposure of 1100 mGy.[119] It is estimated that the additional radiation from diagnostic X-rays will increase the average person's cumulative risk of getting cancer by age 75 by 0.6–3.0%.[120] The amount of absorbed radiation depends upon the type of X-ray test and the body part involved.[116] CT and fluoroscopy entail higher doses of radiation than do plain X-rays.
To place the increased risk in perspective, a plain chest X-ray will expose a person to the same amount from background radiation that people are exposed to (depending upon location) every day over 10 days, while exposure from a dental X-ray is approximately equivalent to 1 day of environmental background radiation.[121] Each such X-ray would add less than 1 per 1,000,000 to the lifetime cancer risk. An abdominal or chest CT would be the equivalent to 2–3 years of background radiation to the whole body, or 4–5 years to the abdomen or chest, increasing the lifetime cancer risk between 1 per 1,000 to 1 per 10,000.[121] This is compared to the roughly 40% chance of a US citizen developing cancer during their lifetime.[122] For instance, the effective dose to the torso from a CT scan of the chest is about 5 mSv, and the absorbed dose is about 14 mGy.[123] A head CT scan (1.5 mSv, 64 mGy)[124] that is performed once with and once without contrast agent, would be equivalent to 40 years of background radiation to the head. Accurate estimation of effective doses due to CT is difficult with the estimation uncertainty range of about ±19% to ±32% for adult head scans depending upon the method used.[125]
The risk of radiation is greater to a fetus, so in pregnant patients, the benefits of the investigation (X-ray) should be balanced with the potential hazards to the fetus.[126][127] If there is 1 scan in 9 months, it can be harmful to the fetus.[128] Therefore, women who are pregnant get ultrasounds as their diagnostic imaging because this does not use radiation.[128] If there is too much radiation exposure there could be harmful effects on the fetus or the reproductive organs of the mother.[128] In the US, there are an estimated 62 million CT scans performed annually, including more than 4 million on children.[116] Avoiding unnecessary X-rays (especially CT scans) reduces radiation dose and any associated cancer risk.[129]
Medical X-rays are a significant source of human-made radiation exposure. In 1987, they accounted for 58% of exposure from human-made sources in the United States. Since human-made sources accounted for only 18% of the total radiation exposure, most of which came from natural sources (82%), medical X-rays only accounted for 10% of total American radiation exposure; medical procedures as a whole (including nuclear medicine) accounted for 14% of total radiation exposure. By 2006, however, medical procedures in the United States were contributing much more ionizing radiation than was the case in the early 1980s. In 2006, medical exposure constituted nearly half of the total radiation exposure of the U.S. population from all sources. The increase is traceable to the growth in the use of medical imaging procedures, in particular computed tomography (CT), and to the growth in the use of nuclear medicine.[104][130]

Dosage due to dental X-rays varies significantly depending on the procedure and the technology (film or digital). Depending on the procedure and the technology, a single dental X-ray of a human results in an exposure of 5 to 40 μSv. A full mouth series of X-rays may result in an exposure of up to 60 (digital) to 180 (film) μSv, for a yearly average of up to 400 μSv.[131][132][133][134][135][136][137]
Financial incentives have been shown to have a significant impact on X-ray use with doctors who are paid a separate fee for each X-ray providing more X-rays.[138]
Early photon tomography or EPT[139] (as of 2015) along with other techniques[140] are being researched as potential alternatives to X-rays for imaging applications.
Other uses
[edit]Other notable uses of X-rays include:

- X-ray crystallography in which the pattern produced by the diffraction of X-rays through the closely spaced lattice of atoms in a crystal is recorded and then analysed to reveal the nature of that lattice. A related technique, fiber diffraction, was used by Rosalind Franklin to discover the double helical structure of DNA.[141]
- X-ray astronomy, which is an observational branch of astronomy, which deals with the study of X-ray emission from celestial objects.
- X-ray microscopic analysis, which uses electromagnetic radiation in the soft X-ray band to produce images of very small objects.
- X-ray fluorescence, a technique in which X-rays are generated within a specimen and detected. The outgoing energy of the X-ray can be used to identify the composition of the sample.
- Industrial radiography uses X-rays for inspection of industrial parts, particularly welds.
- Radiography of cultural objects, most often X-rays of paintings to reveal underdrawing, pentimenti alterations in the course of painting or by later restorers, and sometimes previous paintings on the support. Many pigments such as lead white show well in radiographs.
- X-ray spectromicroscopy has been used to analyse the reactions of pigments in paintings. For example, in analysing colour degradation in the paintings of van Gogh.[142]

- Authentication and quality control of packaged items.
- Industrial CT (computed tomography), a process that uses X-ray equipment to produce three-dimensional representations of components both externally and internally. This is accomplished through computer processing of projection images of the scanned object in many directions.
- Airport security luggage scanners use X-rays for inspecting the interior of luggage for security threats before loading on aircraft.
- Border control truck scanners and domestic police departments use X-rays for inspecting the interior of trucks.

- X-ray art and fine art photography, artistic use of X-rays, for example the works by Stane Jagodič
- X-ray hair removal, a method popular in the 1920s but now banned by the FDA.[144]
- Shoe-fitting fluoroscopes were popularized in the 1920s, banned in the US in the 1960s, in the UK in the 1970s, and later in continental Europe.
- Roentgen stereophotogrammetry is used to track movement of bones based on the implantation of markers
- X-ray photoelectron spectroscopy is a chemical analysis technique relying on the photoelectric effect, usually employed in surface science.
- Radiation implosion is the use of high energy X-rays generated from a fission explosion (an A-bomb) to compress nuclear fuel to the point of fusion ignition (an H-bomb).
Visibility
[edit]While generally considered invisible to the human eye, in special circumstances X-rays can be visible. Brandes, in an experiment a short time after Röntgen's landmark 1895 paper, reported after dark adaptation and placing his eye close to an X-ray tube, seeing a faint "blue-gray" glow which seemed to originate within the eye itself.[145] Upon hearing this, Röntgen reviewed his record books and found he too had seen the effect. When placing an X-ray tube on the opposite side of a wooden door Röntgen had noted the same blue glow, seeming to emanate from the eye itself, but thought his observations to be spurious because he only saw the effect when he used one type of tube. Later he realized that the tube which had created the effect was the only one powerful enough to make the glow plainly visible and the experiment was thereafter readily repeatable. The knowledge that X-rays are actually faintly visible to the dark-adapted naked eye has largely been forgotten today; this is probably due to the desire not to repeat what would now be seen as a recklessly dangerous and potentially harmful experiment with ionizing radiation. It is not known what exact mechanism in the eye produces the visibility: it could be due to conventional detection (excitation of rhodopsin molecules in the retina), direct excitation of retinal nerve cells, or secondary detection via, for instance, X-ray induction of phosphorescence in the eyeball with conventional retinal detection of the secondarily produced visible light.
Though X-rays are otherwise invisible, it is possible to see the ionization of the air molecules if the intensity of the X-ray beam is high enough. The beamline from the wiggler at the European Synchrotron Radiation Facility[146] is one example of such high intensity.[147]
Units of measure and exposure
[edit]The measure of X-rays ionizing ability is called the exposure:
- The coulomb per kilogram (C/kg) is the SI unit of ionizing radiation exposure, and it is the amount of radiation required to create one coulomb of charge of each polarity in one kilogram of matter.
- The roentgen (R) is an obsolete traditional unit of exposure, which represented the amount of radiation required to create one electrostatic unit of charge of each polarity in one cubic centimeter of dry air. 1 roentgen = 2.58×10−4 C/kg.
However, the effect of ionizing radiation on matter (especially living tissue) is more closely related to the amount of energy deposited into them rather than the charge generated. This measure of energy absorbed is called the absorbed dose:
- The gray (Gy), which has units of (joules/kilogram), is the SI unit of absorbed dose, and it is the amount of radiation required to deposit one joule of energy in one kilogram of any kind of matter.
- The rad is the (obsolete) corresponding traditional unit, equal to 10 millijoules of energy deposited per kilogram. 100 rad = 1 gray.
The equivalent dose is the measure of the biological effect of radiation on human tissue. For X-rays it is equal to the absorbed dose.
- The Roentgen equivalent man (rem) is the traditional unit of equivalent dose. For X-rays it is equal to the rad, or, in other words, 10 millijoules of energy deposited per kilogram. 100 rem = 1 Sv.
- The sievert (Sv) is the SI unit of equivalent dose, and also of effective dose. For X-rays the "equivalent dose" is numerically equal to a Gray (Gy). 1 Sv = 1 Gy. For the "effective dose" of X-rays, it is usually not equal to the Gray (Gy).
| Quantity | Unit | Symbol | Derivation | Year | SI equivalent |
|---|---|---|---|---|---|
| Activity (A) | becquerel | Bq | s−1 | 1974 | SI unit |
| curie | Ci | 3.7×1010 s−1 | 1953 | 3.7×1010 Bq | |
| rutherford | Rd | 106 s−1 | 1946 | 1000000 Bq | |
| Exposure (X) | coulomb per kilogram | C/kg | C⋅kg−1 of air | 1974 | SI unit |
| röntgen | R | esu / 0.001293 g of air | 1928 | 2.58×10−4 C/kg | |
| Absorbed dose (D) | gray | Gy | J⋅kg−1 | 1974 | SI unit |
| erg per gram | erg/g | erg⋅g−1 | 1950 | 1.0×10−4 Gy | |
| rad | rad | 100 erg⋅g−1 | 1953 | 0.010 Gy | |
| Equivalent dose (H) | sievert | Sv | J⋅kg−1 × WR | 1977 | SI unit |
| röntgen equivalent man | rem | 100 erg⋅g−1 × WR | 1971 | 0.010 Sv | |
| Effective dose (E) | sievert | Sv | J⋅kg−1 × WR × WT | 1977 | SI unit |
| röntgen equivalent man | rem | 100 erg⋅g−1 × WR × WT | 1971 | 0.010 Sv |
See also
[edit]- Backscatter X-ray – Advanced X-ray imaging technology
- Detective quantum efficiency – Quality metric for imaging detectors
- High-energy X-rays – X-ray with energies between 80 and 1000 kiloelectronvolts
- Macintyre's X-Ray Film – 1896 film by John Macintyre
- N ray – Hypothetical form of radiation
- Neutron radiation – Ionizing radiation that presents as free neutrons
- NuSTAR – NASA X-ray space telescope of the Explorer program
- Radiographer – Healthcare professional
- Reflection (physics) – "Bouncing back" of waves at an interface
- Resonant inelastic X-ray scattering – Advanced X-ray spectroscopy technique
- Small-angle X-ray scattering – Radiation scattering technique
- The X-Rays – 1897 British film by George Albert Smith
- X-ray absorption spectroscopy – Spectroscopic technique
- X-ray marker
- X-ray nanoprobe
- X-ray reflectivity – Surface analytical technique
- X-ray vision – Fictional superpower
- X-ray welding
References
[edit]- ^ "Figure 7.1, Wavelengths and frequencies of the different groups of electromagnetic radiation. X-rays lie in the range of 0.01 nm up to 10 nm - Medical Imaging Systems - NCBI Bookshelf". ncbi.nlm.nih.gov. 3 August 2018. Retrieved 8 October 2024.
- ^ "X-Rays". Science Mission Directorate. NASA.
- ^ Novelline, Robert (1997). Squire's Fundamentals of Radiology. Harvard University Press. 5th edition. ISBN 0-674-83339-2.
- ^ Dental Assistants' Association of Australia (2005). The Manual of Dental Assisting. Elsevier Australia. p. 205. ISBN 978-0-7295-3737-7.
- ^ Caldwell, Wallace E.; Merrill, Edward H. (1964). History of the World. Vol. 1. United States: The Greystone Press. p. 394.
- ^ Filler A (2009). "The History, Development and Impact of Computed Imaging in Neurological Diagnosis and Neurosurgery: CT, MRI, and DTI". Nature Precedings. doi:10.1038/npre.2009.3267.4.
- ^ Morgan W (24 February 1785). "Electrical Experiments Made in Order to Ascertain the Non-Conducting Power of a Perfect Vacuum, &c". Philosophical Transactions of the Royal Society. 75. Royal Society of London: 272–278. doi:10.1098/rstl.1785.0014.
- ^ Anderson JG (January 1945). "William Morgan and X-rays". Transactions of the Faculty of Actuaries. 17: 219–221. doi:10.1017/s0071368600003001.
- ^ Thomson JJ (1903). The Discharge of Electricity through Gasses. US: Charles Scribner's Sons. pp. 182–186.
- ^ Wiedmann's Annalen, Vol. XLVIII.
- ^ Walden, T L (December 1991). "The first radiation accident in America: a centennial account of the x-ray photograph made in 1890". Radiology. 181 (3): 635–639. doi:10.1148/radiology.181.3.1947073. ISSN 0033-8419. PMID 1947073.
- ^ http://www.smj.org.sg/sites/default/files/3605/3605hdxray1.pdf
- ^ a b Illustrated Electrical Review: A Journal of Scientific and Electrical Progress. Electrical Review Publishing Company. 1894.
- ^ Wyman T (Spring 2005). "Fernando Sanford and the Discovery of X-rays". "Imprint", from the Associates of the Stanford University Libraries: 5–15.
- ^ a b Hrabak M, Padovan RS, Kralik M, Ozretic D, Potocki K (July 2008). "Scenes from the past: Nikola Tesla and the discovery of X-rays". Radiographics. 28 (4): 1189–1192. doi:10.1148/rg.284075206. PMID 18635636.
- ^ Chadda PK (2009). Hydroenergy and Its Energy Potential. Pinnacle Technology. p. 88. ISBN 978-1-61820-149-2.
- ^ Tesla's technical publications indicate that he invented and developed a single-electrode X-ray tube. Morton, William James and Hammer, Edwin W. (1896) American Technical Book Co., p. 68. U.S. patent 514,170, "Incandescent Electric Light". U.S. patent 454,622 "System of Electric Lighting". These differed from other X-ray tubes in having no target electrode and worked with the output of a Tesla coil.
- ^ Stanton A (23 January 1896). "Wilhelm Conrad Röntgen On a New Kind of Rays: translation of a paper read before the Würzburg Physical and Medical Society, 1895". Nature. 53 (1369): 274–6. Bibcode:1896Natur..53R.274.. doi:10.1038/053274b0. see also pp. 268 and 276 of the same issue.
- ^ Garcia, J.; Buchwald, N. A.; Feder, B. H.; Koelling, R. A.; Tedrow, L. (1964). "Sensitivity of the head to X-ray". Science. 144 (3625): 1470–1472. Bibcode:1964Sci...144.1470G. doi:10.1126/science.144.3625.1470. ISSN 0036-8075. PMID 14171545. S2CID 44719943.
Rats have been trained to respond to signals consisting of very low doses of chi-ray directed to the head.
- ^ Baganha, M. F.; Marques, M. A.; Botelho, M. F.; Teixeira, M. L.; Carvalheira, V.; Calisto, J.; Silva, A.; Fernandes, A.; Torres, M.; Brito, J. (1993). "Tomodensitometry and radioisotopic methods in the study of unilateral lung hyperlucencies of vascular origin". Acta Médica Portuguesa. 6 (1): 19–24. ISSN 0870-399X. PMID 8475784.
- ^ Takahashi, K.; Case, B. W.; Dufresne, A.; Fraser, R.; Higashi, T.; Siemiatycki, J. (1994). "Relation between lung asbestos fibre burden and exposure indices based on job history". Occupational and Environmental Medicine. 51 (7): 461–469. doi:10.1136/oem.51.7.461. ISSN 1351-0711. PMC 1128015. PMID 8044245.
- ^ Peters P (1995). "W. C. Roentgen and the discovery of x-rays". Textbook of Radiology. Medcyclopedia.com, GE Healthcare. Archived from the original on 11 May 2008. Retrieved 5 May 2008.
- ^ Glasser O (1993). Wilhelm Conrad Röntgen and the early history of the roentgen rays. Norman Publishing. pp. 10–15. ISBN 978-0930405229.
- ^ Arthur C (8 November 2010). "Google doodle celebrates 115 years of X-rays". The Guardian. Guardian US. Retrieved 5 February 2019.
- ^ Kevles BH (1996). Naked to the Bone Medical Imaging in the Twentieth Century. Camden, New Jersey: Rutgers University Press. pp. 19–22. ISBN 978-0-8135-2358-3.
- ^ Sample S (27 March 2007). "X-Rays". The Electromagnetic Spectrum. NASA. Retrieved 3 December 2007.
- ^ Markel H (20 December 2012). "'I Have Seen My Death': How the World Discovered the X-Ray". PBS NewsHour. PBS. Retrieved 23 March 2019.
- ^ Glasser O (1958). Dr. W. C. Ro ̈ntgen. Springfield: Thomas.
- ^ Natale S (1 November 2011). "The Invisible Made Visible". Media History. 17 (4): 345–358. doi:10.1080/13688804.2011.602856. hdl:2134/19408. S2CID 142518799.
- ^ Natale S (4 August 2011). "A Cosmology of Invisible Fluids: Wireless, X-Rays, and Psychical Research Around 1900". Canadian Journal of Communication. 36 (2): 263–276. doi:10.22230/cjc.2011v36n2a2368. hdl:2318/1770480.
- ^ Grove AW (1 January 1997). "Röntgen's ghosts: photography, X-rays, and the Victorian imagination". Literature and Medicine. 16 (2): 141–173. doi:10.1353/lm.1997.0016. PMID 9368224. S2CID 35604474.
- ^ Karlsson EB (9 February 2000). "The Nobel Prizes in Physics 1901–2000". Stockholm: The Nobel Foundation. Retrieved 24 November 2011.
- ^ a b c d e f Feldman A (November 1989). "A sketch of the technical history of radiology from 1896 to 1920". Radiographics. 9 (6): 1113–1128. doi:10.1148/radiographics.9.6.2685937. PMID 2685937.
- ^ "Major John Hall-Edwards". Birmingham City Council. Archived from the original on 28 September 2012. Retrieved 17 May 2012.
- ^ Kudriashov, Y. B. (2008). Radiation Biophysics. Nova Publishers. p. xxi. ISBN 9781600212802.
- ^ "Green, James (Zoological Artist), Sciagraphs of British batrachians and reptiles, 1897". Yale Centre for British Art. Retrieved 24 November 2021.
- ^ "Sciagraphs of British Batrachians and Reptiles1". Nature. 55 (1432): 539–540. 1 April 1897. Bibcode:1897Natur..55..539.. doi:10.1038/055539a0. S2CID 4054184.
- ^ a b Spiegel PK (January 1995). "The first clinical X-ray made in America—100 years". AJR. American Journal of Roentgenology. 164 (1): 241–243. doi:10.2214/ajr.164.1.7998549. PMID 7998549.
- ^ Nicolaas A. Rupke, Eminent Lives in Twentieth-Century Science and Religion, page 300, Peter Lang, 2009 ISBN 3631581203
- ^ "Visible Proofs: Forensic Views of the Body: Galleries: Cases: Could X-rays Have Saved President William McKinley?". NLM.NIH.gov. Retrieved 24 January 2022.
- ^ Daniel J (April 1896). "THE X-RAYS". Science. 3 (67): 562–563. Bibcode:1896Sci.....3..562D. doi:10.1126/science.3.67.562. PMID 17779817.
- ^ Fleming WL (1909). The South in the Building of the Nation: Biography A-J. Pelican Publishing. p. 300. ISBN 978-1589809468.
- ^ Ce4Rt (March 2014). Understanding Ionizing Radiation and Protection. p. 174.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ Glasser O (1934). Wilhelm Conrad Röntgen and the Early History of the Roentgen Rays. Norman Publishing. p. 294. ISBN 978-0930405229.
- ^ Sansare K, Khanna V, Karjodkar F (February 2011). "Early victims of X-rays: a tribute and current perception". Dento Maxillo Facial Radiology. 40 (2): 123–125. doi:10.1259/dmfr/73488299. PMC 3520298. PMID 21239576.
- ^ a b "ISU Health Physics Radinf – First 50 Years". Sites.Google.com. Retrieved 24 January 2022.
- ^ California, San Francisco Area Funeral Home Records, 1835–1979. Database with images. FamilySearch. Jacob Fleischman in the entry for Elizabeth Aschheim. 3 August 1905. Citing funeral home J.S. Godeau, San Francisco, San Francisco, California. Record book Vol. 06, p. 1–400, 1904–1906. San Francisco Public Library. San Francisco History and Archive Center.
- ^ Editor. (5 August 1905). Aschheim. Obituaries. San Francisco Examiner. San Francisco, California.
- ^ Editor. (5 August 1905). Obituary Notice. Elizabeth Fleischmann. San Francisco Chronicle. Page 10.
- ^ "Major John Hall-Edwards". Birmingham City Council. Archived from the original on 28 September 2012. Retrieved 23 April 2010.
- ^ "JOHN HALL-EDWARDS". Engole the Elven for Knowledge. 15 June 2018. Retrieved 27 October 2023.
- ^ a b Schall K (1905). Electro-medical Instruments and their Management. Bemrose & Sons Ltd. Printers. pp. 96, 107.
- ^ Stoddart C (1 March 2022). "Structural biology: How proteins got their close-up". Knowable Magazine. doi:10.1146/knowable-022822-1. Retrieved 25 March 2022.
- ^ Birmingham City Council: Major John Hall-Edwards Archived 28 September 2012 at the Wayback Machine
- ^ "X-ray movies show hard boiled egg fighting digestive organs (1913)". The News-Palladium. 4 April 1913. p. 2. Retrieved 26 November 2020.
- ^ "X-ray moving pictures latest (1913)". Chicago Tribune. 22 June 1913. p. 32. Retrieved 26 November 2020.
- ^ "Homeopaths to show movies of body's organs at work (1915)". The Central New Jersey Home News. 10 May 1915. p. 6. Retrieved 26 November 2020.
- ^ "How X-Ray Movies Are Taken (1918)". Davis County Clipper. 15 March 1918. p. 2. Retrieved 26 November 2020.
- ^ "X-ray movies (1919)". Tampa Bay Times. 12 January 1919. p. 16. Retrieved 26 November 2020.
- ^ "X-ray movies perfected. Will show motions of bones and joints of human body. (1918)". The Sun. 7 January 1918. p. 7. Retrieved 26 November 2020.
- ^ "Talk is cheap? X-ray used by Institute of Phonetics (1920)". New Castle Herald. 2 January 1920. p. 13. Retrieved 26 November 2020.
- ^ Jorgensen TJ (10 October 2017). "Marie Curie and her X-ray vehicles' contribution to World War I battlefield medicine". The Conversation. Retrieved 23 February 2018.
- ^ "X-Rays for Fitting Boots". Warwick Daily News (Qld.: 1919–1954). 25 August 1921. p. 4. Retrieved 27 November 2020.
- ^ "T. C. BEIRNE'S X-RAY SHOE FITTING". Telegraph (Brisbane, Qld. : 1872–1947). 17 July 1925. p. 8. Retrieved 5 November 2017.
- ^ "THE PEDOSCOPE". Sunday Times (Perth, WA : 1902–1954). 15 July 1928. p. 5. Retrieved 5 November 2017.
- ^ "X-RAY SHOE FITTINGS". Biz (Fairfield, NSW : 1928–1972). 27 July 1955. p. 10. Retrieved 5 November 2017.
- ^ "SHOE X-RAY DANGERS". Brisbane Telegraph (Qld. : 1948–1954). 28 February 1951. p. 7. Retrieved 5 November 2017.
- ^ "X-ray shoe sets in S.A. 'controlled'". News (Adelaide, SA : 1923–1954). 27 April 1951. p. 12. Retrieved 5 November 2017.
- ^ "Ban On Shoe X-ray Machines Resented". Canberra Times (ACT : 1926–1995). 26 June 1957. p. 4. Retrieved 5 November 2017.
- ^ Fitzgerald R (2000). "Phase-sensitive x-ray imaging". Physics Today. 53 (7): 23–26. Bibcode:2000PhT....53g..23F. doi:10.1063/1.1292471. S2CID 121322301.
- ^ a b David C, Nöhammer B, Solak H, Ziegler (2002). "Differential x-ray phase contrast imaging using a shearing interferometer". Applied Physics Letters. 81 (17): 3287–3289. Bibcode:2002ApPhL..81.3287D. doi:10.1063/1.1516611.
- ^ Wilkins SW, Gureyev TE, Gao D, Pogany A, Stevenson AW (1996). "Phase-contrast imaging using polychromatic hard X-rays". Nature. 384 (6607): 335–338. Bibcode:1996Natur.384..335W. doi:10.1038/384335a0. S2CID 4273199.
- ^ Davis TJ, Gao D, Gureyev TE, Stevenson AW, Wilkins SW (1995). "Phase-contrast imaging of weakly absorbing materials using hard X-rays". Nature. 373 (6515): 595–598. Bibcode:1995Natur.373..595D. doi:10.1038/373595a0. S2CID 4287341.
- ^ Momose A, Takeda T, Itai Y, Hirano K (April 1996). "Phase-contrast X-ray computed tomography for observing biological soft tissues". Nature Medicine. 2 (4): 473–475. doi:10.1038/nm0496-473. PMID 8597962. S2CID 23523144.
- ^ Attwood, David (1999). Soft X-rays and extreme ultraviolet radiation. Cambridge University. p. 2. ISBN 978-0-521-65214-8. Archived from the original on 11 November 2012. Retrieved 4 November 2012.
- ^ "Physics.nist.gov". Physics.nist.gov. Retrieved 8 November 2011.
- ^ a b Denny PP, Heaton B (1999). Physics for Diagnostic Radiology. US: CRC Press. p. 12. ISBN 978-0-7503-0591-4.
- ^ Feynman R, Leighton R, Sands M (1963). The Feynman Lectures on Physics. Vol. 1. US: Addison-Wesley. pp. 2–8. ISBN 978-0-201-02116-5.
- ^ L'Annunziata M, Abrade M (2003). Handbook of Radioactivity Analysis. Academic Press. p. 58. ISBN 978-0-12-436603-9.
- ^ Grupen C, Cowan G, Eidelman SD, Stroh T (2005). Astroparticle Physics. Springer. p. 109. ISBN 978-3-540-25312-9.
- ^ Hodgman, Charles, ed. (1961). CRC Handbook of Chemistry and Physics, 44th Ed. US: Chemical Rubber Co. p. 2850.
- ^ Government of Canada, Canadian Centre for Occupational Health and Safety (9 May 2019). "Radiation – Quantities and Units of Ionizing Radiation: OSH Answers". CCOHS.ca. Retrieved 9 May 2019.
- ^ Bushberg, Jerrold T.; Seibert, J. Anthony; Leidholdt, Edwin M.; Boone, John M. (2002). The essential physics of medical imaging. Lippincott Williams & Wilkins. p. 42. ISBN 978-0-683-30118-2.
- ^ Bushberg, Jerrold T.; Seibert, J. Anthony; Leidholdt, Edwin M.; Boone, John M. (2002). The essential physics of medical imaging. Lippincott Williams & Wilkins. p. 38. ISBN 978-0-683-30118-2.
- ^ Kissel L (2 September 2000). "RTAB: the Rayleigh scattering database". Radiation Physics and Chemistry. 59 (2). Lynn Kissel: 185–200. Bibcode:2000RaPC...59..185K. doi:10.1016/S0969-806X(00)00290-5. Archived from the original on 12 December 2011. Retrieved 8 November 2012.
- ^ Attwood, David (1999). "3". Soft X-rays and extreme ultraviolet radiation. Cambridge University Press. ISBN 978-0-521-65214-8. Archived from the original on 11 November 2012. Retrieved 4 November 2012.
- ^ "X-ray Transition Energies Database". NIST Physical Measurement Laboratory. 9 December 2011. Retrieved 19 February 2016.
- ^ "X-Ray Data Booklet Table 1-3" (PDF). Center for X-ray Optics and Advanced Light Source, Lawrence Berkeley National Laboratory. 1 October 2009. Archived from the original (PDF) on 23 April 2009. Retrieved 19 February 2016.
- ^ Whaites E, Cawson R (2002). Essentials of Dental Radiography and Radiology. Elsevier Health Sciences. pp. 15–20. ISBN 978-0-443-07027-3.
- ^ Bushburg J, Seibert A, Leidholdt E, Boone J (2002). The Essential Physics of Medical Imaging. US: Lippincott Williams & Wilkins. p. 116. ISBN 978-0-683-30118-2.
- ^ Emilio B, Ballerna A (1994). "Preface". Biomedical Applications of Synchrotron Radiation: Proceedings of the 128th Course at the International School of Physics -Enrico Fermi- 12–22 July 1994, Varenna, Italy. IOS Press. p. xv. ISBN 90-5199-248-3.
- ^ Camara CG, Escobar JV, Hird JR, Putterman SJ (2008). "Correlation between nanosecond X-ray flashes and stick–slip friction in peeling tape" (PDF). Nature. 455 (7216): 1089–1092. Bibcode:2008Natur.455.1089C. doi:10.1038/nature07378. S2CID 4372536. Retrieved 2 February 2013.
- ^ Paul H, Muhr J (1986). "Review of experimental cross sections for K-shell ionization by light ions". Physics Reports. 135 (2): 47–97. Bibcode:1986PhR...135...47P. doi:10.1016/0370-1573(86)90149-3.
- ^ Köhn C, Ebert U (2014). "Angular distribution of Bremsstrahlung photons and of positrons for calculations of terrestrial gamma-ray flashes and positron beams". Atmospheric Research. 135–136: 432–465. arXiv:1202.4879. Bibcode:2014AtmRe.135..432K. doi:10.1016/j.atmosres.2013.03.012. S2CID 10679475.
- ^ Köhn C, Ebert U (2015). "Calculation of beams of positrons, neutrons, and protons associated with terrestrial gamma ray flashes". Journal of Geophysical Research: Atmospheres. 120 (4): 1620–1635. Bibcode:2015JGRD..120.1620K. doi:10.1002/2014JD022229.
- ^ Kochkin P, Köhn C, Ebert U, Van Deursen L (May 2016). "Analyzing x-ray emissions from meter-scale negative discharges in ambient air". Plasma Sources Science and Technology. 25 (4): 044002. Bibcode:2016PSST...25d4002K. doi:10.1088/0963-0252/25/4/044002. S2CID 43609721.
- ^ Cooray V, Arevalo L, Rahman M, Dwyer J, Rassoul H (2009). "On the possible origin of X-rays in long laboratory sparks". Journal of Atmospheric and Solar-Terrestrial Physics. 71 (17–18): 1890–1898. Bibcode:2009JASTP..71.1890C. doi:10.1016/j.jastp.2009.07.010.
- ^ Köhn C, Chanrion O, Neubert T (March 2017). "Electron acceleration during streamer collisions in air". Geophysical Research Letters. 44 (5): 2604–2613. Bibcode:2017GeoRL..44.2604K. doi:10.1002/2016GL072216. PMC 5405581. PMID 28503005.
- ^ Köhn C, Chanrion O, Babich LP, Neubert T (2018). "Streamer properties and associated x-rays in perturbed air". Plasma Sources Science and Technology. 27 (1): 015017. Bibcode:2018PSST...27a5017K. doi:10.1088/1361-6595/aaa5d8.
- ^ Köhn C, Chanrion O, Neubert T (May 2018). "High-Energy Emissions Induced by Air Density Fluctuations of Discharges". Geophysical Research Letters. 45 (10): 5194–5203. Bibcode:2018GeoRL..45.5194K. doi:10.1029/2018GL077788. PMC 6049893. PMID 30034044.
- ^ Förster A, Brandstetter S, Schulze-Briese C (June 2019). "Transforming X-ray detection with hybrid photon counting detectors". Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences. 377 (2147): 20180241. Bibcode:2019RSPTA.37780241F. doi:10.1098/rsta.2018.0241. PMC 6501887. PMID 31030653.
- ^ Thomas, Adrian M.K. (August 2007). "The first 50 years of military radiology 1895–1945". European Journal of Radiology. 63 (2): 214–219. doi:10.1016/j.ejrad.2007.05.024. PMID 17629432.
- ^ a b Roobottom CA, Mitchell G, Morgan-Hughes G (November 2010). "Radiation-reduction strategies in cardiac computed tomographic angiography". Clinical Radiology. 65 (11): 859–867. doi:10.1016/j.crad.2010.04.021. PMID 20933639.
Of the 5 billion imaging investigations performed worldwide...
- ^ a b "Medical Radiation Exposure Of The U.S. Population Greatly Increased Since The Early 1980s". ScienceDaily. Retrieved 24 January 2022.
- ^ Rhinehart, D. A. (December 1931). "Air and Gas in the Soft Tissues: A Radiologic Study". Radiology. 17 (6): 1158–1170. doi:10.1148/17.6.1158. ISSN 0033-8419.
- ^ Van Haver A, Kolk S, DeBoodt S, Valkering K, Verdonk P (2018). "Accuracy of total knee implant position assessment based on postoperative X-rays, registered to pre-operative CT-based 3D models". Orthopaedic Proceedings. 99-B (Supp 4).
- ^ Herman GT (2009). Fundamentals of Computerized Tomography: Image Reconstruction from Projections (2nd ed.). Springer. ISBN 978-1-85233-617-2.
- ^ a b Hermena, Shady; Young, Michael (2024), "CT-scan Image Production Procedures", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 34662062, retrieved 20 April 2024
- ^ a b Davros, William J. (1 April 2007). "Fluoroscopy: basic science, optimal use, and patient/operator protection". Techniques in Regional Anesthesia and Pain Management. Imaging for Interventional Management of Chronic Pain. 11 (2): 44–54. doi:10.1053/j.trap.2007.02.005. ISSN 1084-208X.
- ^ Advances in kilovoltage x-ray beam dosimetry in Hill R, Healy B, Holloway L, Kuncic Z, Thwaites D, Baldock C (March 2014). "Advances in kilovoltage x-ray beam dosimetry". Physics in Medicine and Biology. 59 (6): R183–R231. Bibcode:2014PMB....59R.183H. doi:10.1088/0031-9155/59/6/r183. PMID 24584183. S2CID 18082594.
- ^ Thwaites DI, Tuohy JB (July 2006). "Back to the future: the history and development of the clinical linear accelerator". Physics in Medicine and Biology. 51 (13): R343–R362. Bibcode:2006PMB....51R.343T. doi:10.1088/0031-9155/51/13/R20. PMID 16790912. S2CID 7672187.
- ^ "11th Report on Carcinogens". Ntp.niehs.nih.gov. Archived from the original on 9 December 2010. Retrieved 8 November 2010.
- ^ Hall EJ, Brenner DJ (May 2008). "Cancer risks from diagnostic radiology". The British Journal of Radiology. 81 (965): 362–378. doi:10.1259/bjr/01948454. PMID 18440940.
- ^ Brenner DJ (2010). "Should we be concerned about the rapid increase in CT usage?". Reviews on Environmental Health. 25 (1): 63–68. doi:10.1515/REVEH.2010.25.1.63. PMID 20429161. S2CID 17264651.
- ^ De Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF, Caruso A (September 2007). "Radiation effects on development". Birth Defects Research. Part C, Embryo Today. 81 (3): 177–182. doi:10.1002/bdrc.20099. PMID 17963274.
- ^ a b c Brenner DJ, Hall EJ (November 2007). "Computed tomography—an increasing source of radiation exposure". The New England Journal of Medicine. 357 (22): 2277–2284. doi:10.1056/NEJMra072149. PMID 18046031. S2CID 2760372.
- ^ Upton AC (July 2003). "The state of the art in the 1990's: NCRP Report No. 136 on the scientific bases for linearity in the dose-response relationship for ionizing radiation". Health Physics. 85 (1): 15–22. doi:10.1097/00004032-200307000-00005. PMID 12852466. S2CID 13301920.
- ^ Calabrese EJ, Baldwin LA (February 2003). "Toxicology rethinks its central belief" (PDF). Nature. 421 (6924): 691–692. Bibcode:2003Natur.421..691C. doi:10.1038/421691a. PMID 12610596. S2CID 4419048. Archived from the original (PDF) on 12 September 2011.
- ^ Oakley PA, Ehsani NN, Harrison DE (1 April 2019). "The Scoliosis Quandary: Are Radiation Exposures From Repeated X-Rays Harmful?". Dose-Response. 17 (2): 1559325819852810. doi:10.1177/1559325819852810. PMC 6560808. PMID 31217755.
- ^ Berrington de González A, Darby S (January 2004). "Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries". Lancet. 363 (9406): 345–351. doi:10.1016/S0140-6736(04)15433-0. PMID 15070562. S2CID 8516754.
- ^ a b "Radiation Dose in X-Ray and CT Exams". RadiologyInfo.org. Radiological Society of North America (RSNA) and American College of Radiology (ACR). Retrieved 24 January 2022.
- ^ "National Cancer Institute: Surveillance Epidemiology and End Results (SEER) data". Seer.cancer.gov. 30 June 2010. Retrieved 8 November 2011.
- ^ Caon M, Bibbo G, Pattison J (2000). "Monte Carlo calculated effective dose to teenage girls from computed tomography examinations". Radiation Protection Dosimetry. 90 (4): 445–448. doi:10.1093/oxfordjournals.rpd.a033172.
- ^ Shrimpton, P.C; Miller, H.C; Lewis, M.A; Dunn, M. Doses from Computed Tomography (CT) examinations in the UK – 2003 Review Archived 22 September 2011 at the Wayback Machine
- ^ Gregory KJ, Bibbo G, Pattison JE (August 2008). "On the Uncertainties in Effective Dose Estimates of Adult CT Head Scans". Medical Physics. 35 (8): 3501–3510. Bibcode:2008MedPh..35.3501G. doi:10.1118/1.2952359. PMID 18777910.
- ^ Giles D, Hewitt D, Stewart A, Webb J (September 1956). "Malignant disease in childhood and diagnostic irradiation in utero". Lancet. 271 (6940): 447. doi:10.1016/S0140-6736(56)91923-7. PMID 13358242.
- ^ "Pregnant Women and Radiation Exposure". eMedicine Live online medical consultation. Medscape. 28 December 2008. Archived from the original on 23 January 2009. Retrieved 16 January 2009.
- ^ a b c Ratnapalan S, Bentur Y, Koren G (December 2008). ""Doctor, will that x-ray harm my unborn child?"". CMAJ. 179 (12): 1293–1296. doi:10.1503/cmaj.080247. PMC 2585137. PMID 19047611.
- ^ Donnelly LF (February 2005). "Reducing radiation dose associated with pediatric CT by decreasing unnecessary examinations". AJR. American Journal of Roentgenology. 184 (2): 655–657. doi:10.2214/ajr.184.2.01840655. PMID 15671393.
- ^ US National Research Council (2006). Health Risks from Low Levels of Ionizing Radiation, BEIR 7 phase 2. National Academies Press. pp. 5, fig.PS–2. ISBN 978-0-309-09156-5., data credited to NCRP (US National Committee on Radiation Protection) 1987
- ^ "ANS / Public Information / Resources / Radiation Dose Calculator". Archived from the original on 16 May 2012. Retrieved 16 May 2012.
- ^ "HOW DANGEROUS IS RADIATION?". PhyAst.Pitt.edu. Retrieved 24 January 2022.
- ^ Muller, Richard. Physics for Future Presidents, Princeton University Press, 2010
- ^ X-Rays Archived 15 March 2007 at the Wayback Machine. Doctorspiller.com (9 May 2007). Retrieved on 2011-05-05.
- ^ X-Ray Safety Archived 4 April 2007 at the Wayback Machine. Dentalgentlecare.com (6 February 2008). Retrieved on 2011-05-05.
- ^ "Dental X-Rays". Idaho State University. Archived from the original on 7 November 2012. Retrieved 7 November 2012.
- ^ D.O.E. – About Radiation Archived 27 April 2012 at the Wayback Machine
- ^ Chalkley M, Listl S (March 2018). "First do no harm – The impact of financial incentives on dental X-rays". Journal of Health Economics. 58 (March 2018): 1–9. doi:10.1016/j.jhealeco.2017.12.005. hdl:2066/190628. PMID 29408150.
- ^ "Using lasers instead of x-rays". Open University. 24 February 2011. Retrieved 28 July 2021.
- ^ Dent S (12 February 2015). "Scientists achieve X-ray vision with safe, visible light". Engadget. Retrieved 28 July 2021.
- ^ Kasai N, Kakudo, M (2005). X-ray diffraction by macromolecules. Tokyo: Kodansha. pp. 291–2. ISBN 978-3-540-25317-4.
- ^ Monico L, Van der Snickt G, Janssens K, De Nolf W, Miliani C, Verbeeck J, et al. (February 2011). "Degradation process of lead chromate in paintings by Vincent van Gogh studied by means of synchrotron X-ray spectromicroscopy and related methods. 1. Artificially aged model samples". Analytical Chemistry. 83 (4): 1214–1223. doi:10.1021/ac102424h. PMID 21314201. Monico L, Van der Snickt G, Janssens K, De Nolf W, Miliani C, Dik J, et al. (February 2011). "Degradation process of lead chromate in paintings by Vincent van Gogh studied by means of synchrotron X-ray spectromicroscopy and related methods. 2. Original paint layer samples". Analytical Chemistry. 83 (4): 1224–1231. doi:10.1021/ac1025122. PMID 21314202.
- ^ Ahi K, Anwar M (May 2016). "Advanced terahertz techniques for quality control and counterfeit detection". In Anwar MF, Crowe TW, Manzur T (eds.). Terahertz Physics, Devices, and Systems X: Advanced Applications in Industry and Defense. Vol. 9856. Society of Photographic Instrumentation Engineers. pp. 31–44.
- ^ Bickmore, Helen (2003). Milady's Hair Removal Techniques: A Comprehensive Manual. Thomson Delmar Learning. ISBN 978-1401815554.
- ^ Frame P. "Wilhelm Röntgen and the Invisible Light". Tales from the Atomic Age. Oak Ridge Associated Universities. Retrieved 11 October 2021.
- ^ European Synchrotron Radiation Facility ID11
- ^ Als-Nielsen, Jens; Mcmorrow, Des (2001). Elements of Modern X-Ray Physics. John Wiley & Sons Ltd. pp. 40–41. ISBN 978-0-471-49858-2.
External links
[edit]- "On a New Kind of Rays". Nature. 53 (1369): 274–276. January 1896. Bibcode:1896Natur..53R.274.. doi:10.1038/053274b0.
- "Ion X-Ray tubes". The Cathode Ray Tube site.
- "Index of Early Bremsstrahlung Articles". Shade Tree Physics. 12 April 2010.
- Samuel JJ (20 October 2013). "La découverte des rayons X par Röntgen". Bibnum Education (in French). Röntgen's discovery of X-rays (PDF; English translation)
- Oakley, P. A., Navid Ehsani, N., & Harrison, D. E. (2020). 5 Reasons Why Scoliosis X-Rays Are Not Harmful. Dose-Response. https://doi.org/10.1177/1559325820957797
- "X-ray Crystallography". A web for learning how X-rays can "see" inside the crystals.